Different electrodes have different standard electrode potentials. The standard electrode potentials for some electrodes are negative, while for some others it is positive. The potential values of many electrodes have been measured experimentally and their standard reduction potentials are arranged in sequential order. This arrangement of elements in order of increasing reduction potential values is called electrochemical series or galvanic series.
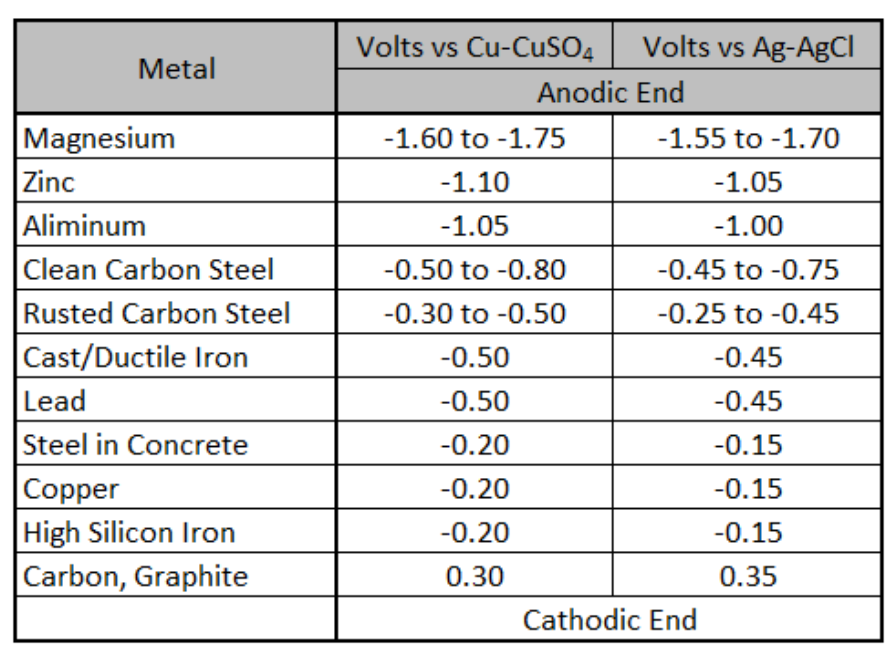
The potential value of an electrode gives the relative tendency for the reduction reaction to occur at the electrode as compared to that of the reduction of H+ ion under standard conditions. Therefore, the electrodes with positive electrode potentials show a greater tendency towards reduction than the H+. While the electrodes with negative electrode potentials show the lesser tendency towards reduction than the tendency of H+ ions.
Read Also: Cathodic Protection